- Department of Pediatric Neurosurgery, Advocate Children’s Hospital, Park Ridge,
- Department of Neurological Surgery, Loyola University Medical Center, Maywood, Illinois,
- Department of Neurosurgery, Children’s Hospital of the King’s Daughters, Norfolk, Virginia,
- Department of Pediatrics, Loyola University Medical Center, Maywood,
- Department of Neurosurgery, Carle Foundation Hospital, Urbana, Illinois, United States.
Correspondence Address:
John Ta-Hsiang Tsiang, Department of Neurological Surgery, Loyola University Medical Center, Maywood, Illinois, United States.
DOI:10.25259/SNI_1158_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daphne Li1,2, John Ta-Hsiang Tsiang2, Kimberly A. Mackey3, Andrew Bonwit4, Suguna Pappu5. Cephalohematomas, an occult nidus for infection and inflammation: A case report and review of the literature. 03-Feb-2023;14:38
How to cite this URL: Daphne Li1,2, John Ta-Hsiang Tsiang2, Kimberly A. Mackey3, Andrew Bonwit4, Suguna Pappu5. Cephalohematomas, an occult nidus for infection and inflammation: A case report and review of the literature. 03-Feb-2023;14:38. Available from: https://surgicalneurologyint.com/surgicalint-articles/12142/
Abstract
Background: Cephalohematomas (CH) are benign neonatal fluid collections that arise between the periosteum and skull due to birth trauma, and usually resolve spontaneously without intervention. CH may rarely become infected.
Case Description: The authors report a case of sterile CH requiring surgical evacuation in a persistently febrile neonate treated with intravenous (IV) antibiotics for Escherichia coli urosepsis. Diagnostic tap of the CH yielded no pathogens, but given the persistence of fevers, surgical evacuation was performed. The patient demonstrated clinical improvement postoperatively.
Conclusion: A systematic review of literature was conducted through a MEDLINE search using the keyword “cephalohematoma.” Articles were screened for cases of infected CH and their subsequent management. Clinicopathological characteristics and outcomes of the present case were reviewed and compared to those in the literature. Infected CH were reported in 25 articles describing 58 patients. Common pathogens included E. coli and Staphylococcal species. Treatment included a course of IV antibiotics (10 days–6 weeks) and often included percutaneous aspiration (n = 47) for diagnostic and therapeutic purposes. Surgical evacuation was performed in 23 cases. To the authors’ knowledge, the present case is the first documented report in which evacuation of a culture-negative CH resulted in resolution of the patient’s clinical symptoms of sepsis that persisted despite appropriate antibiotic treatment. This suggests that patients with CH should be evaluated through diagnostic tap of the collection if there are signs of local or persistent systemic infection. Surgical evacuation may be indicated if percutaneous aspiration does not result in clinical improvement.
Keywords: Birth trauma, Cephalohematoma, Infection, Pediatric neurosurgery
INTRODUCTION
Cephalohematomas (CHs) occur in 1–4% of live-births, often associated with birth trauma.[
METHODS
A systematic MEDLINE search was conducted using the keyword “cephalohematoma.” The reference lists from these publications were examined for relevant literature and historical reports [
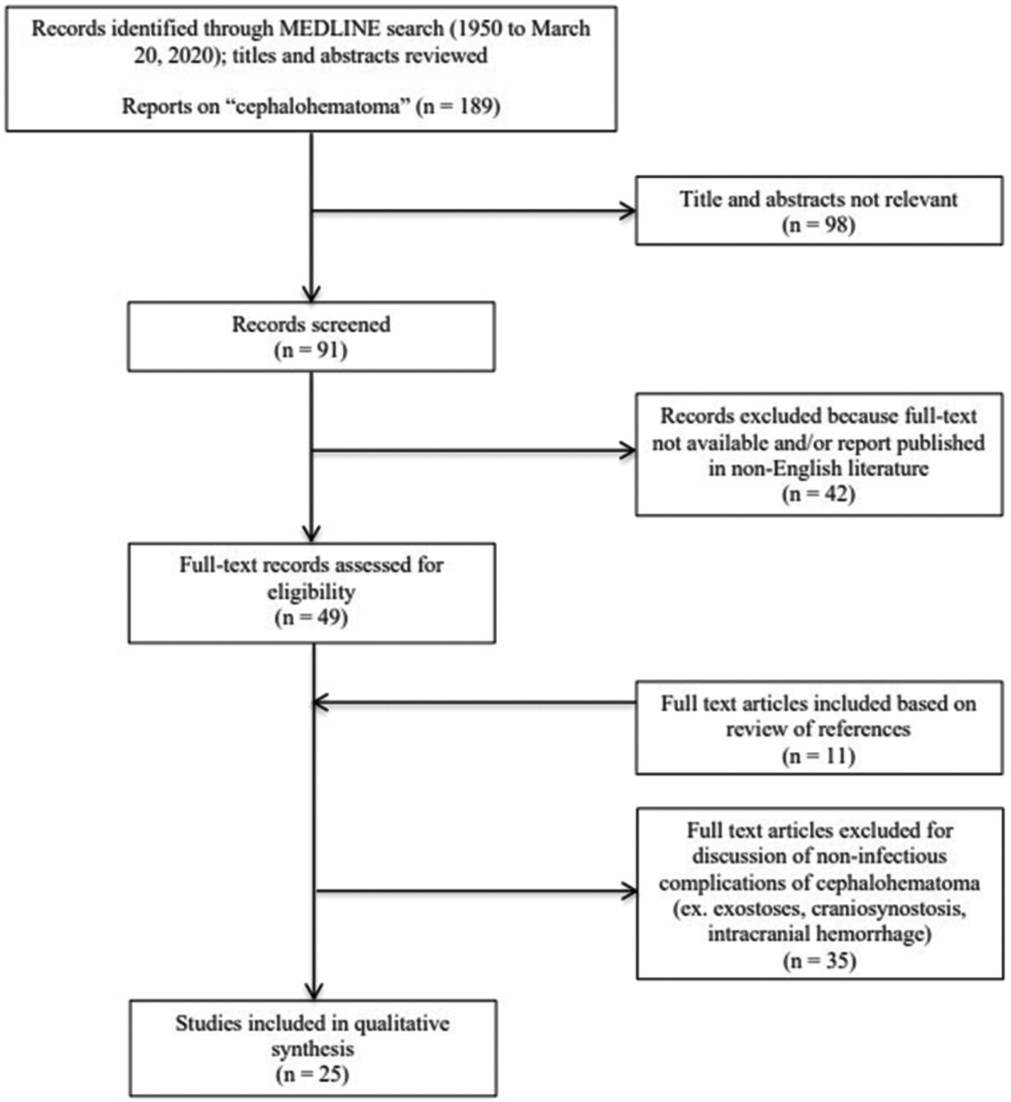
Figure 1:
PRISMA flow diagram. Citations identified and evaluated on literature review of infected cephalohematomas. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/ journal.pmed1000097
Clinicopathological characteristics and outcomes of the present case were compared to those in the literature. The patient’s chart was reviewed for demographic data, clinical presentation, hospital course, relevant imaging, and follow-up using our electronic medical record system. Clinical case review was deemed not human subjects research and exempt from approval by our Institutional Review Board.
CASE DESCRIPTION
A male infant was born at term through uncomplicated vaginal delivery without instrument-assistance or fetal scalp electrodes. A left-sided CH was noted post-partum. At 3 weeks old, he presented to an outside institution with 1-day of fever, emesis, and diarrhea. He was started empirically on IV ampicillin and gentamicin, and infectious workup later revealed pan-sensitive E. coli bacteremia, urinary tract infection, and meningitis. After initial clinical improvement, he was transitioned to monotherapy with IV ampicillin. Shortly thereafter, he became febrile again, so cultures were redrawn and a head ultrasound performed, which was largely unrevealing. The patient was transferred to our institution for further management [
On arrival, he was noted to have a large, benign-appearing CH, and a reassuring physical examination, despite being febrile with a rising C-reactive protein (CRP). Magnetic resonance imaging (MRI) of the brain did not reveal any acute intracranial processes or definitive signs of CH infection [
Figure 2:
(a-h) Magnetic resonance imaging (MRI) study of the patient’s brain on presentation to our institution. Select axial T2-weighted non-contrast (a), T1-weighted non-contrast (b), T1-weighted post-contrast (c), gradient echo (d), diffusion-weighted imaging (e), apparent diffusion coefficient (f), sagittal T1-weighted non-contrast (g), and coronal T1-weighted non-contrast (h) images from an MRI study of the patient’s brain. Images demonstrate a large heterogeneous extracranial fluid collection overlying the left parietal skull consistent with a cephalohematoma. The collection does have some enhancement along its margins, but does not demonstrate restricted diffusion. No significant surrounding subcutaneous edema is identified. No acute intracranial findings or abnormalities noted.
Figure 3:
Documentation of patient’s vital signs during his admission at our institution. Patient’s body maximum temperature in °C (a) and median recorded heart rate (beats per minute; bpm) (b), as charted per nursing documentation for each 24-h period throughout the course of the patient’s admission. Patient continued to be intermittently febrile (>38°C or 100.4°F; red line) and tachycardic (>160 bpm; red line) until after the patient was taken to the operating room for surgical I&D of the cephalohematoma, on his 7th day of admission to our institution (grey rectangle).
Our patient became afebrile on postoperative day (POD)1 and remained clinically improved for the remainder of his hospitalization [
Literature review
A MEDLINE search was conducted using the keyword “cephalohematoma.” Full-text, English-language articles were reviewed for management and outcomes of infected CH. This resulted in 25 publications, documenting 58 cases [
The majority of cases 52% (n = 30) had one or more concurrent infections,[
All patients were treated with IV antibiotics; some followed by a course of oral antibiotics. Duration varied from 10 days to 6 weeks, depending on concerns for osteomyelitis or other concurrent infections. The majority were treated with needle aspiration alone (n = 34/58).[
DISCUSSION
CH infection is rare and may occur early (<2 weeks of life) due to direct birth trauma or hematogenous seeding, or late (≥3 weeks of life) due to overlying cellulitis.[
Aspiration and surgical interventions are contraindicated in uncomplicated CH due to risks of contamination with bacteria.[
The presented case makes a unique contribution to the body of literature because this patient’s CH did not demonstrate evidence of infection on any diagnostic modality. It may be argued that because our patient received IV antibiotics for several days before needle aspiration or I&D, the CH may have been sterilized by appropriate treatment of pan-sensitive E. coli. There has been growing evidence for polymerase chain reaction testing of culture-negative fluid collections that are suspected to have harbored bacterial infections following initiating of empiric antibiotics. This test was not performed for our patient, but may be helpful to reveal a causative organism.[
Although the exact mechanism is unclear, the authors believe that CH evacuation was still indicated in this case given our patient’s persistent signs of systemic infection.
CONCLUSION
Optimal management of infected CH often requires a combination of IV antibiotics and evacuation. In the absence of clear local signs of infection, patients demonstrating a relapse or persistent signs of systemic infection should still be considered for definitive CH evacuation, even with negative cultures.
Ethics approval/consent to participate
Due to its retrospective nature, clinical case review was deemed not human subjects research and exempt from approval by the Loyola University Chicago Stritch School of Medicine Institutional Review Board (LU#213917) on July 22, 2020. Therefore, the Loyola University Chicago Stritch School of Medicine Institutional Review Board has waived the need for the patient’s and/or parents’/legal guardian’s written informed consent for publication. All included patient information has been deidentified.
Author contributions
Daphne Li is the first author having performed the majority of data extraction, literature review, data analysis, and writing of the manuscript. John Tsiang contributed to data analysis and drafting of the manuscript. John Tsiang, Kimberly Mackey, and Andrew Bonwit contributed to the critical review of the manuscript. Suguna Pappu is the principal investigator having contributed to the majority of design and review of the manuscript. All authors read and approved the final manuscript.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Blom NA, Vreede WB. Infected cephalhematomas associated with osteomyelitis, sepsis and meningitis. Pediatr Infect Dis J. 1993. 12: 1015-7
2. Brook I. Infected neonatal cephalohematomas caused by anaerobic bacteria. J Perinat Med. 2005. 33: 255-8
3. Chang HY, Chiu NC, Huang FY, Kao HA, Hsu CH, Hung HY. Infected cephalohematoma of newborns: Experience in a medical center in Taiwan. Pediatr Int. 2005. 47: 274-7
4. Chen MH, Yang JC, Huang JS, Chen MH. MRI features of an infected cephalhaematoma in a neonate. J Clin Neurosci. 2006. 13: 849-52
5. Chmel H, Palmer JA, Eikman EA. Soft tissue hematoma as a cause of fever in the adult. Diagn Microbiol Infect Dis. 1988. 11: 215-9
6. Dahl KM, Barry J, DeBiasi RL. Escherichia hermannii infection of a cephalohematoma: Case report, review of the literature, and description of a novel invasive pathogen. Clin Infect Dis. 2002. 35: e96-8
7. Ellis SS, Montgomery JR, Wagner M, Hill RM. Osteomyelitis complicating neonatal cephalhematoma. Am J Dis Child. 1974. 127: 100-2
8. Farrell JJ, Wang H, Sampath R, Lowery KS, Bonomo RA. The effect of empiric antimicrobial treatment duration on detection of bacterial DNA in sterile surgical specimens. PLoS One. 2017. 12: e0171074
9. Goodwin MD, Persing JA, Duncan CC, Shin JH. Spontaneously infected cephalohematoma: Case report and review of the literature. J Craniofac Surg. 2000. 11: 371-5
10. Hegde HR. Infected cephalhematoma associated with placement of scalp electrode. Can Med Assoc J. 1980. 122: 876-8
11. Hu T, Liu Q, Xu Q, Liu H, Feng Y, Qiu W. Absorption fever characteristics due to percutaneous renal biopsy-related hematoma. Medicine (Baltimore). 2016. 95: e4754
12. Kersten CM, Moellering CM, Mato S. Spontaneous drainage of neonatal cephalohematoma: A delayed complication of scalp abscess. Clin Pediatr (Phila). 2008. 47: 183-5
13. LeBlanc CM, Allen UD, Ventureyra E. Cephalhematomas revisited. When should a diagnostic tap be performed? Clin Pediatr (Phila). 1995. 34: 86-9
14. Lee Y, Berg RB. Cephalhematoma infected with Bacteroides. Am J Dis Child. 1971. 121: 77-8
15. Levy HL, O’Connor JF, Ingall D. Bacteremia, infected cephalhematoma, and osteomyelitis of the skull in a newborn. Am J Dis Child. 1967. 114: 649-51
16. Listinsky JL, Wood BP, Ekholm SE. Parietal osteomyelitis and epidural abscess: A delayed complication of fetal monitoring. Pediatr Radiol. 1986. 16: 150-1
17. Liu H, Li Z, Yang L, Yang X, Zhang Y, Chen J. A 9-day-old neonate with giant scalp abscess: A case report. Medicine (Baltimore). 2019. 98: e17830
18. Malik A, Crowder D, Barretto G, Nield LS, Kiefer A. Subgaleal hematoma masking fatal complications of cephalohematoma in a preterm infant. Clin Pediatr (Phila). 2018. 57: 593-6
19. Mohon RT, Mehalic TF, Grimes CK, Philip AG. Infected cephalhematoma and neonatal osteomyelitis of the skull. Pediatr Infect Dis. 1986. 5: 253-6
20. Nightingale LM, Eaton CB, Fruehan AE, Waldman JB, Clark WB, Lepow ML. Cephalhematoma complicated by osteomyelitis presumed due to Gardnerella vaginalis. JAMA. 1986. 256: 1936-7
21. Nishi J, Kaji T, Tokuda K, Shinkoda Y, Okawa T, Noguchi H. A case of adrenal and cephalhematoma abscesses caused by Escherichia coli after forceps delivery. Pediatr Int. 2005. 47: 480-2
22. Ojumah N, Ramdhan RC, Wilson C, Loukas M, Oskouian RJ, Tubbs RS. Neurological neonatal birth injuries: A literature review. Cureus. 2017. 9: e1938
23. Paul SP, Edate S, Taylor TM. Cephalhaematoma. Infant Dictionary Med Terms. 2009. 5: 146-8
24. Staudt MD, Etarsky D, Ranger A. Infected cephalohematomas and underlying osteomyelitis: A case-based review. Childs Nerv Syst. 2016. 32: 1363-9
25. Vale B, Morais S, Resende C, Taborda A. Neonatal meningitis associated with osteomyelitis and epidural empyema. BMJ Case Rep. 2013. 2013: bcr2013009149
26. Wang JF, Lederhandler MH, Oza VS. Escherichia coli-infected cephalohematoma in an infant. Dermatol Online J. 2018. 24: 13030/qt456083v1
27. Weiss KJ, Edwards MS, Hay LM, Allen CH. Escherichia coli-infected cephalohematoma in an infant. Clin Pediatr (Phila). 2009. 48: 763-6
28. Wong CS, Cheah FC. Cephalhematoma infected by Escherichia coli presenting as an extensive scalp abscess. J Pediatr Surg. 2012. 47: 2336-40
29. Zimmermann P, Duppenthaler A. Infected cephalhaematoma in a five-week-old infant-case report and review of the literature. BMC Infect Dis. 2016. 16: 636